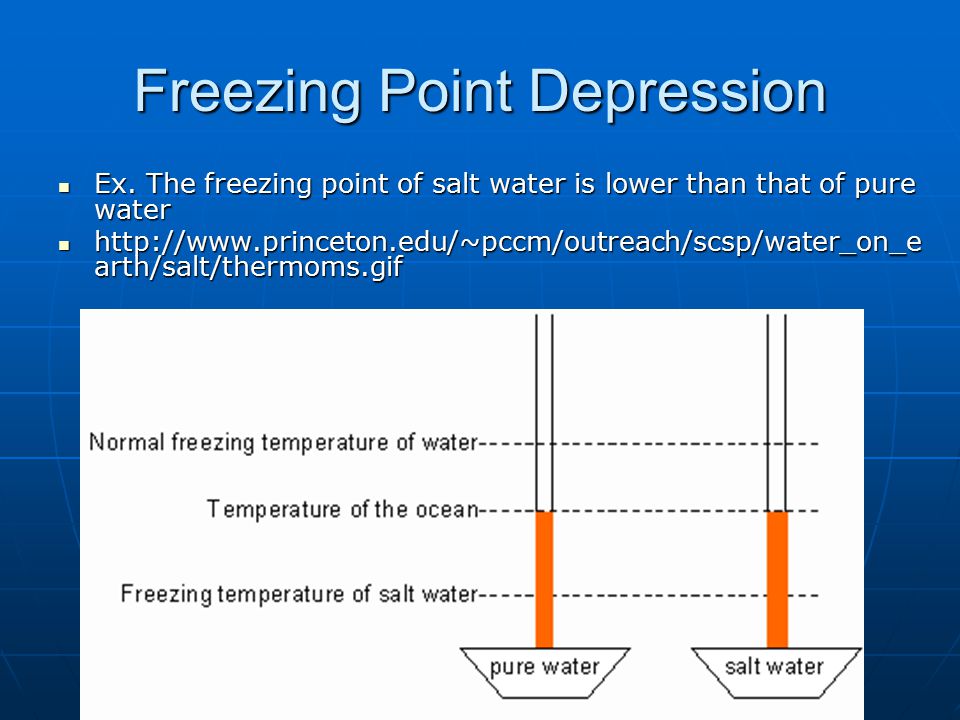
For example when salt is added to water the resulting ions in the water disrupt the usual network of hydrogen bonds made upon freezing. Salt mixed with ice creates a brine that has a temperature lower than 32 f.
Set to 00 0c observe the water molecules in the molecular view.
Freezing temperature of salt water. I know that the freezing point of salt wate. As we all know adding salt to ice water lowers its temperature. So i will try to give you a rough qualitative simplified explanation that howeve.
So if you start out with water that isnt saturated with salt as it freezes the leftover water will get saturated. Thus for un saturated saltwater the freezing happens over a range of temperatures not all at one exact temperature unlike pure. The salt dissolves and the water becomes salty.
Ive read plenty of system level accounts of energy balances enthalpies vapor pressures phase equilibria and freezing pointsi. So saline water remains in a liquid state even at 00c which is the freezing point of normal water and only starts freezing when the temperature lowers furthermost often to 20c and below. So if the water starts to freeze at for example 100c more will freeze as its cooled further until finally the last bit will freeze at 2110c.
With the room temp. This salt water has a lower freezing point so the temperature of the ice bath can get even colder thus freezing the ice cream more. Why does salt lower the freezing point of water.
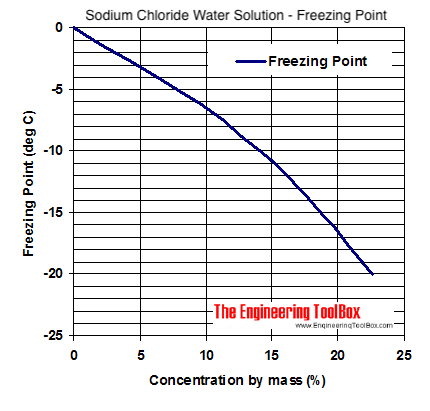
If the water has as much salt dissolved in it as it can hold thats called a saturated solution of salt so that any further salt would just come out as crystals the freezing temperature is around 21 0c or about 6 0f. When salt is added to the ice bath usually rock salt in ice cream making it comes into contact with the thin layer of water on the surface of the melting ice. The freezing point of salt water gizmo shows you how this works.
When salt is added to ice water it lowers melting temperature of ice down to 0 f or so. A correct answer to your question would request a lengthy complex thermodynamic explanation with the use of cumbersome symbols i am not able to reproduce here. If you have a bowl of ice thats melting so the ambient temperature is just above 0 0c what happens to the temperature of the water when you add salt.
Brine is so cold that it easily freezes the ice cream mixture. As a result the freezing point of the solution is lower than it is for the pure solvent. Although pure water freezes at 00c 320f water that has salt dissolved in it has to be colder before it freezes.
When some liquid has same freezing and melting point its liquid and solid forms are said to have a dynamic equilibrium.
 Pure Water Begins To Freeze At Below 0 Celsius B Lower The Room
Pure Water Begins To Freeze At Below 0 Celsius B Lower The Room
Does The Source Of Water Affect The Freezing Point Determined By
When A Salt Water Solution Freezes How Do The Ions Dissolved In
 Chemical Analysis Of Lis Water Dissolved Oxygen What Do Organisms
Chemical Analysis Of Lis Water Dissolved Oxygen What Do Organisms
 Chemistry Department Florida State University
Chemistry Department Florida State University
 Colligative Properties And Phase Diagrams Introductory Chemistry
Colligative Properties And Phase Diagrams Introductory Chemistry
 Freezing Melting And Evaporation
Freezing Melting And Evaporation
 When Salt Is Added To Water Its Freezing Point Lowers What Exact
When Salt Is Added To Water Its Freezing Point Lowers What Exact
 Determining The Molecular Mass By Freezing Point Depression Ppt
Determining The Molecular Mass By Freezing Point Depression Ppt
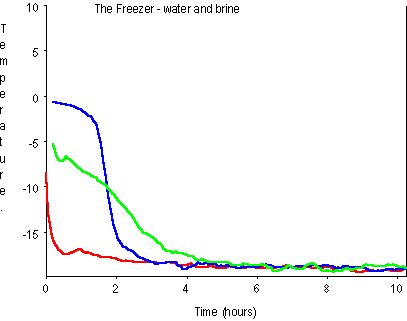
 Brine Freezing Point Chart Heresi Ihi Alliance Org
Brine Freezing Point Chart Heresi Ihi Alliance Org
 To Freeze Or To Melt Water Lab Write Up Malouff S Chemistry Blog
To Freeze Or To Melt Water Lab Write Up Malouff S Chemistry Blog
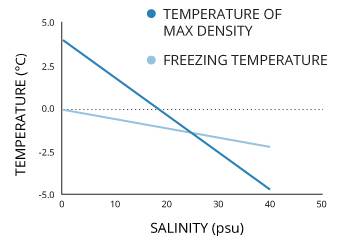 Water Temperature Environmental Measurement Systems
Water Temperature Environmental Measurement Systems
Brine Water Freezing Point Chart Heresi Ihi Alliance Org
Brine Water Freezing Point Chart
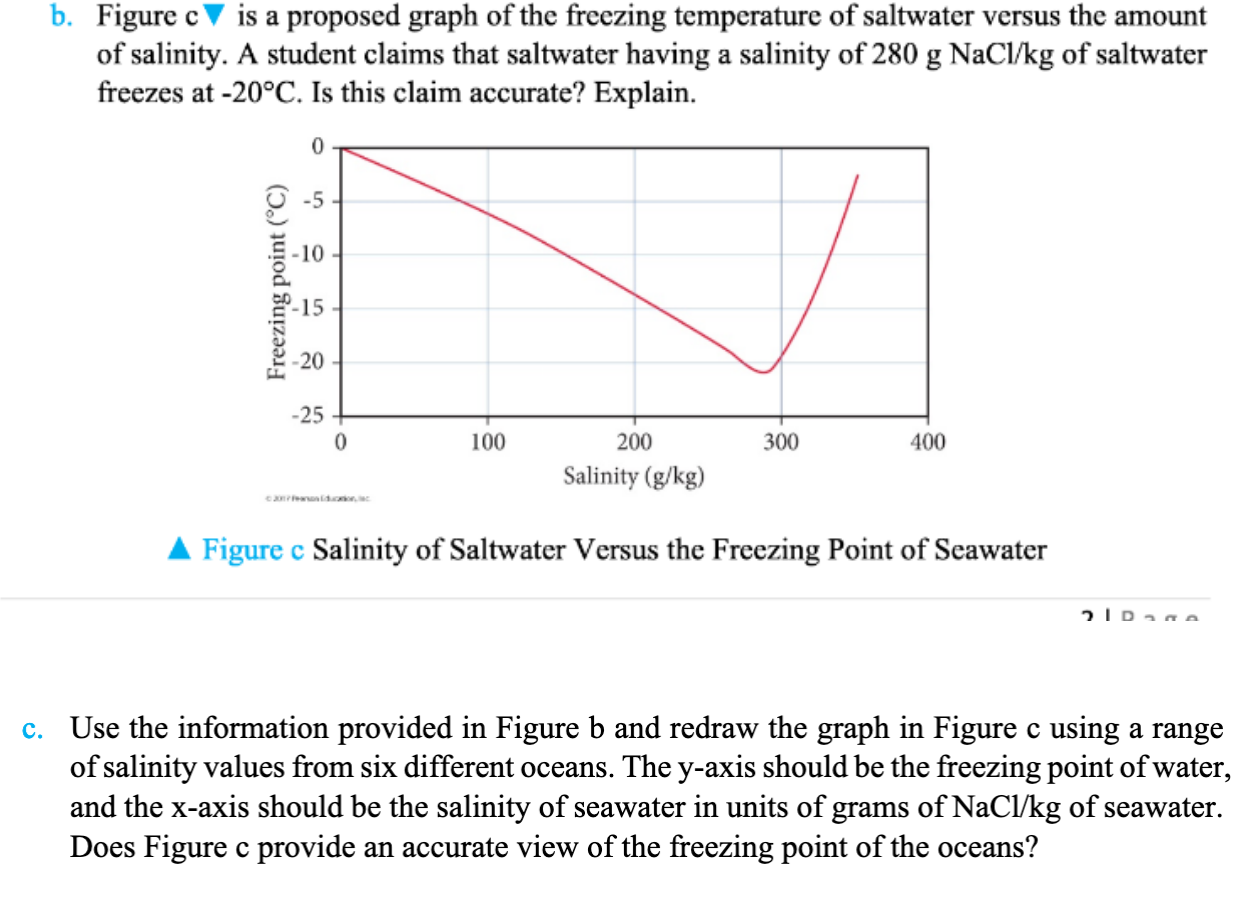 B Figure C Is A Proposed Graph Of The Freezing Te Chegg Com
B Figure C Is A Proposed Graph Of The Freezing Te Chegg Com
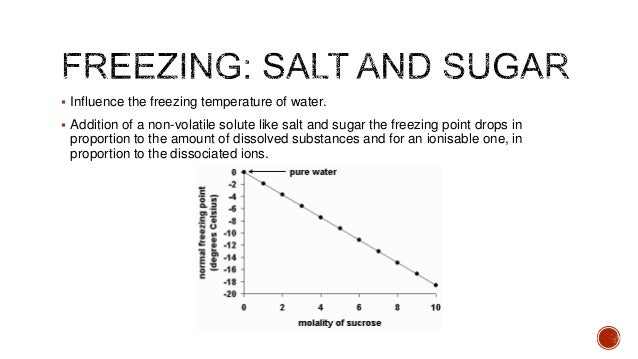
Science Fair Projects The Effect Of Salt And Sugar On The
 If We Add Salt To Ice The Melting Point Decreases What Would
If We Add Salt To Ice The Melting Point Decreases What Would
Why Does The Temperature Of Ice Fall When Some Salt Is Added Quora
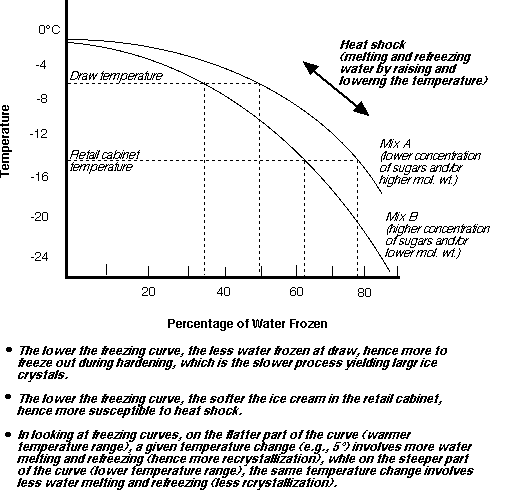
 Crystallization Of Ice In Frozen Desserts
Crystallization Of Ice In Frozen Desserts
Https Arc Nesa Nsw Edu Au Files Science Act6 Ws3 Pdf

Tidak ada komentar:
Posting Komentar