Where mixing occurs with fresh water runoff from river mouths near melting glaciers or vast amounts of precipitation eg. Why does salt lower the freezing point of water.
 Freezing Point Of Salt Water Science Struck
Freezing Point Of Salt Water Science Struck
When salt is added to ice water it lowers melting temperature of ice down to 0 f or so.
Freezing point of saltwater. So in order to know the exact temperature that its going to freeze you have to know just how salty it is. How about with 200 grams of salt. Write your predictions in the appropriate columns below.
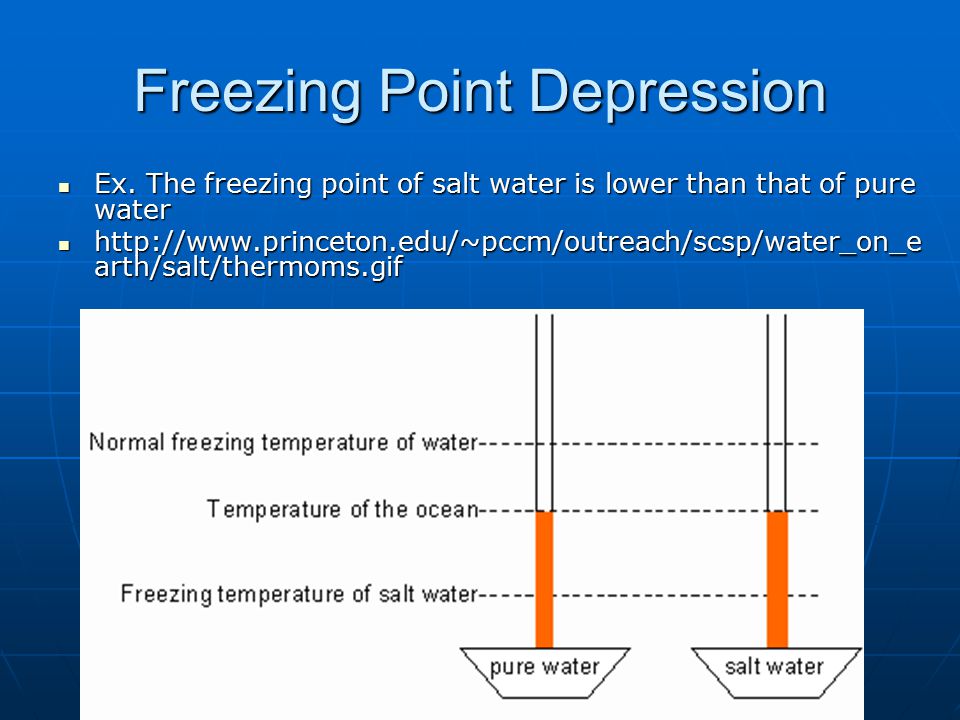
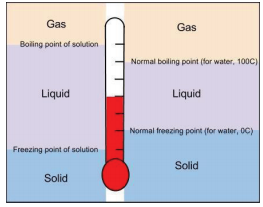
The freezing point of a solution is always less than the freezing point of the pure solvent. Based on your data what do you expect the freezing and melting points to be when 150 grams of salt are added. Theres no way to dissolve any more salt in it no matter how hard you tried the freezing point is 211 degrees celsius.
This means that the solution must be brought to a lower temperature in order for it to freeze. The na and. This also means that melting happens at a lower temperature than for the pure solvent.
Heres a look at the answers to these common questions. The average freezing point of saltwater at normal levels is 28 degrees fahrenheit. Basically the freezing point and boiling point as well of salt water saline water is different from that of freshwater.
For saltwater thats as saturated as it can possibly get ie. Are there any factors that affect the freezing point of water. Monsoon seawater can be substantially less saline.
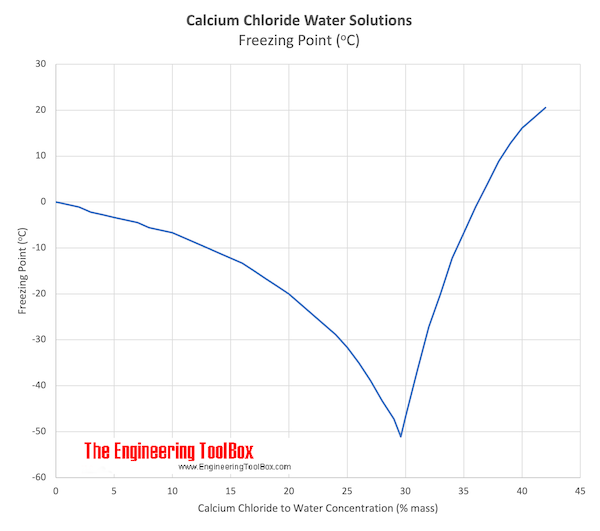
The melting point is lowered by 185 degrees celsius if 292 grams of salt are dissolved in each kg of water called a 05 molal solution of salt. For saltwater the boiling point is raised and the melting point is lowered. In case of polar regions the salinity levels are much low as compared to the same in temperate and tropical regions.
Salt mixed with ice creates a brine that has a temperature lower than 32 f. And the more salt there is in it the lower the freezing point gets. Although the vast majority of seawater has a salinity of between 31 gkg and 38 gkg that is 3138 seawater is not uniformly saline throughout the world.
What is the freezing point of water or the melting point of waterare the freezing point and melting point the same. Brine is so cold that it easily freezes the ice cream mixture. The freezing point of salt water can vary by its salinity.
By how much depends on how much salt there is. How much is the freezing point lowered by adding 50 g of salt. This is when the saltwater is 233 salt by weight.
Ill assume the salt is sodium chloride nacl table salt. Freezing point depression is the decrease of the freezing point of a solvent on the addition of a non volatile soluteexamples include salt in water alcohol in water or the mixing of two solids such as impurities into a finely powdered drug.
 Ice Water Vapor Earth 540 Essentials Of Oceanography For
Ice Water Vapor Earth 540 Essentials Of Oceanography For
 All About Sea Ice National Snow And Ice Data Center
All About Sea Ice National Snow And Ice Data Center
 Zing Point Of Saltwater Gizmo Answers Key Fill Online Printable
Zing Point Of Saltwater Gizmo Answers Key Fill Online Printable
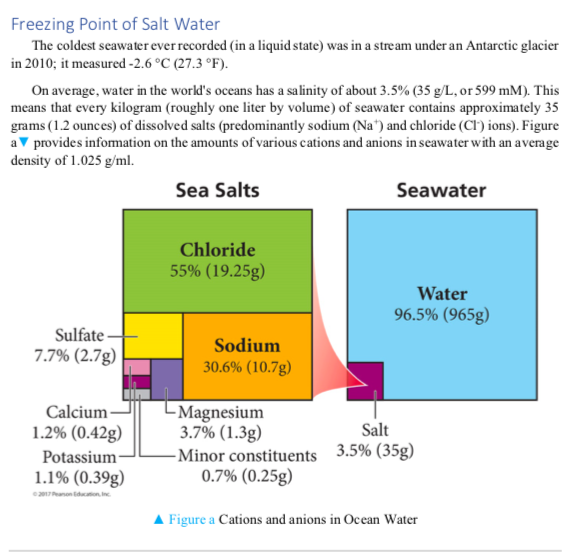
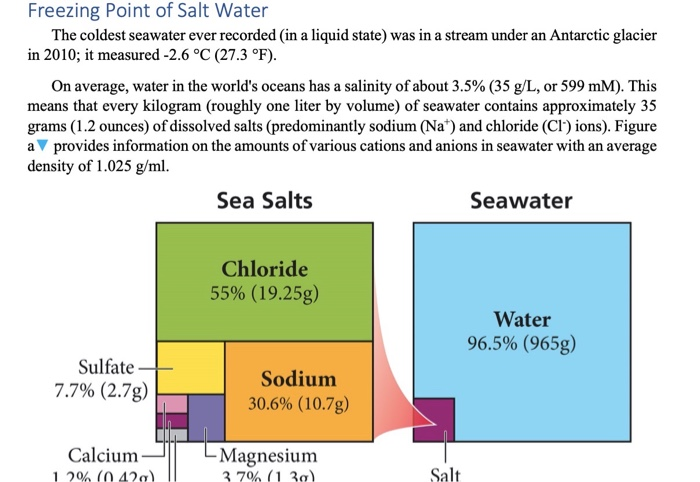
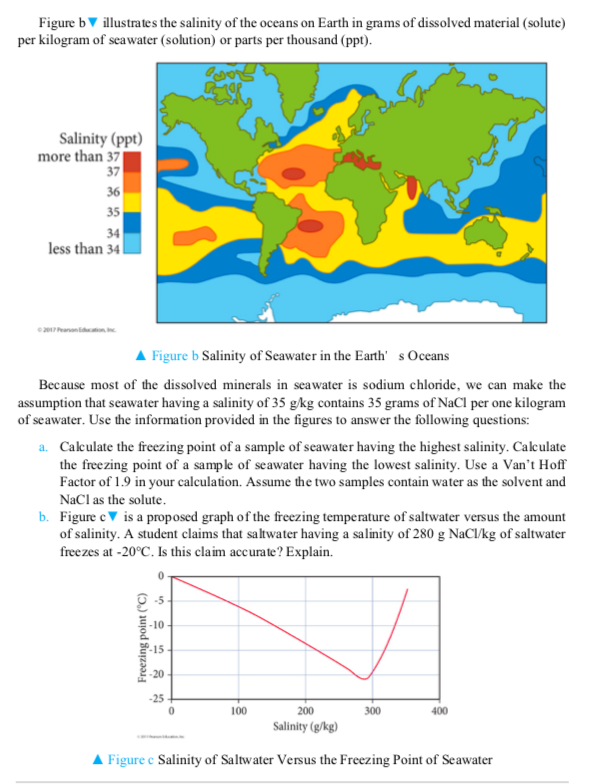 Freezing Point Of Salt Water The Coldest Seawater Chegg Com
Freezing Point Of Salt Water The Coldest Seawater Chegg Com
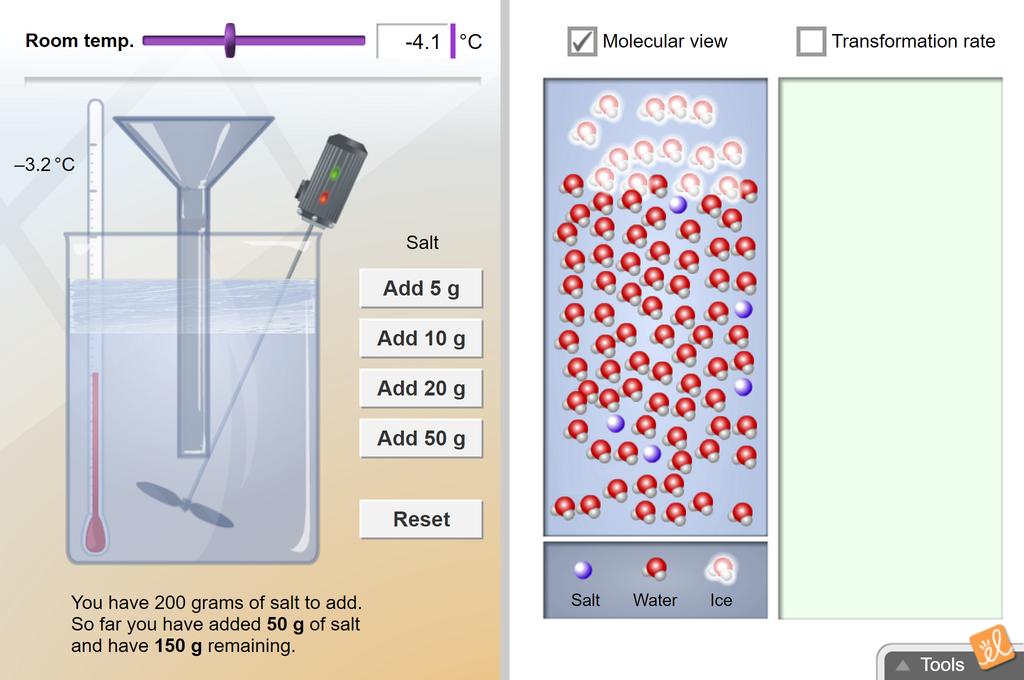 Freezing Point Of Salt Water Gizmo Lesson Info Explorelearning
Freezing Point Of Salt Water Gizmo Lesson Info Explorelearning
 Pdf A Short Report On Action Of Nacl On Freezing Temperature Of
Pdf A Short Report On Action Of Nacl On Freezing Temperature Of
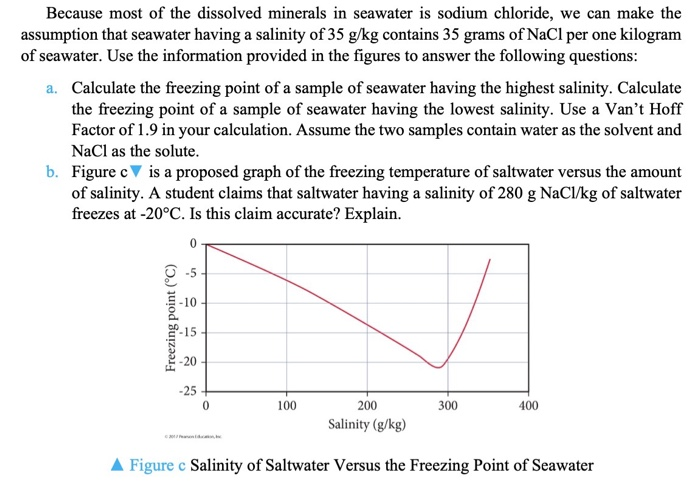 Solved Freezing Point Of Salt Water The Coldest Seawater
Solved Freezing Point Of Salt Water The Coldest Seawater
 Determining The Molecular Mass By Freezing Point Depression Ppt
Determining The Molecular Mass By Freezing Point Depression Ppt
Brine Freezing Point Chart Heresi Ihi Alliance Org
 Which Material Has The Lowest Freezing Point With Picture
Which Material Has The Lowest Freezing Point With Picture
 All About Sea Ice National Snow And Ice Data Center
All About Sea Ice National Snow And Ice Data Center
 Freezing Point Of Salt Water The Coldest Seawater Chegg Com
Freezing Point Of Salt Water The Coldest Seawater Chegg Com
 Solved Freezing Point Of Salt Water The Coldest Seawater
Solved Freezing Point Of Salt Water The Coldest Seawater
 Student Exploration Freezing Point Of Salt Water Answer Key By
Student Exploration Freezing Point Of Salt Water Answer Key By
 Does Salt Water Boil Faster Astrocamp School
Does Salt Water Boil Faster Astrocamp School
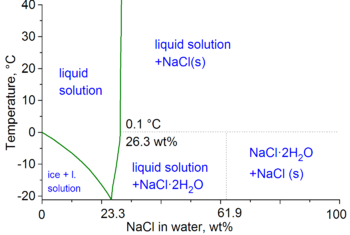
 Phase Diagram Of Nacl H2o Solution 21 Showing The Reduction In
Phase Diagram Of Nacl H2o Solution 21 Showing The Reduction In
 Salt Water Intrusion An Overview Sciencedirect Topics
Salt Water Intrusion An Overview Sciencedirect Topics
Https Arc Nesa Nsw Edu Au Files Science Act6 Ws3 Pdf
 Freezing Point Of Salt Water Freezing Point Of Salt Water Credit
Freezing Point Of Salt Water Freezing Point Of Salt Water Credit
Tidak ada komentar:
Posting Komentar